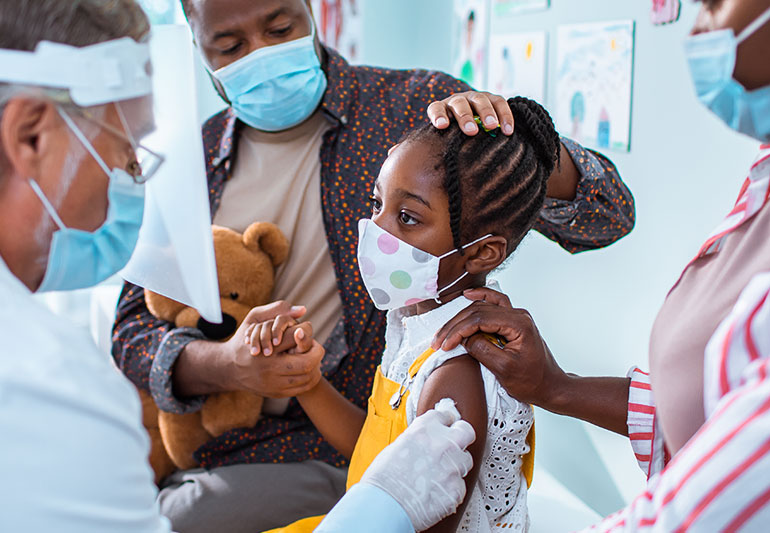
July 2, 2021 — Pfizer plans to request emergency authorization for its COVID-19 vaccine for children ages 5-11 in September or October.
The vaccine is currently authorized for ages 12 and older. Pfizer is now conducting clinical trials in children ages 6 months to 11 years, and the data should be ready to submit to the FDA in the next few months.
“We are planning for emergency use authorization submission for the older age group [ages 5-11] in September or October of this year, and the younger group to follow soon thereafter,” Alejandra Gurtman, MD, Pfizer’s vice president of vaccine clinical research and development, said during a virtual symposium on Wednesday.
Gurtman joined representatives from Moderna and Johnson & Johnson, as well as dozens of public health and vaccine experts, for a discussion of COVID-19 and kids. The symposium was hosted by Johns Hopkins University and the University of Washington.
Pfizer’s pediatric clinical trials are testing lower vaccine doses in younger age groups. Ages 5-11 will receive a 10-microgram dose, and those younger than 5 will receive a 3-microgram dose. About 4,500 children are participating in the trials in the U.S., Finland, Poland, and Spain.
In addition, Moderna is conducting a pediatric clinical trial with 7,000 children between 6 months to 11 years. The company filed for emergency approval of its COVID-19 vaccine for ages 12-17 earlier this month.
“What gets me up in the morning is the goal of having a pediatric vaccine ready to protect children and their communities as soon as safely possible,” Sabine Schnyder Ghamloush, MD, director of clinical development at Moderna, said during the virtual symposium.
Johnson & Johnson will also run four pediatric clinical trials and plans to enroll ages 12-17 this fall before expanding to younger age groups and immunocompromised children.
“As we all know, the pandemic has had a profound impact on children and adolescents,” Macaya Douoguih, MD, head of clinical development and medical affairs at the Janssen Pharmaceutical Companies of Johnson & Johnson, said during the virtual symposium.
“Many have seen close family members suffering or dying from COVID-19 or have experienced complications from the disease themselves, while others have faced interruptions in their education and daily lives when their focus should be on simpler things,” she said. “Collectively, these aspects of the pandemic have had a significant impact on children’s mental and emotional well-being.”
Source: https://www.webmd.com/vaccines/covid-19-vaccine/news/20210702/pfizer-seeking-vaccination-approval-for-ages-5-to-11-years-old-by-fall